Antibodies are glycoproteins, termed as immunoglobulins (Igs), which are produced in response to an immune reaction and specifically bind to antigens responsible for initiating the reaction.
Designua | Shutterstock
Antibody structure
An antibody is composed of two heavy chains (50 KD each) and two light chains (25 KD each), which are joined by disulfide bonds to form a ‘Y’ shaped structure (150 KD). Antibodies are further divided into two regions: a variable region and a constant region.
The variable region is responsible for the antigenic specificity of an antibody. This region includes a fragment antigen binding (Fab) portion that binds the antigen with high specificity. There are two Fab portions in each antibody, which can simultaneously bind two identical epitopes (a specific antibody-binding site of an antigen) of a particular antigen.
The constant region of an antibody includes a fragment crystallization (Fc) portion that binds cell surface receptors (Fc receptors) on circulating WBCs, macrophages, and natural killer cells. This binding is necessary to initiate an immune reaction. In addition, there are two hinge regions that join the Fab and Fc portions of an antibody.
What are the types of antibodies?
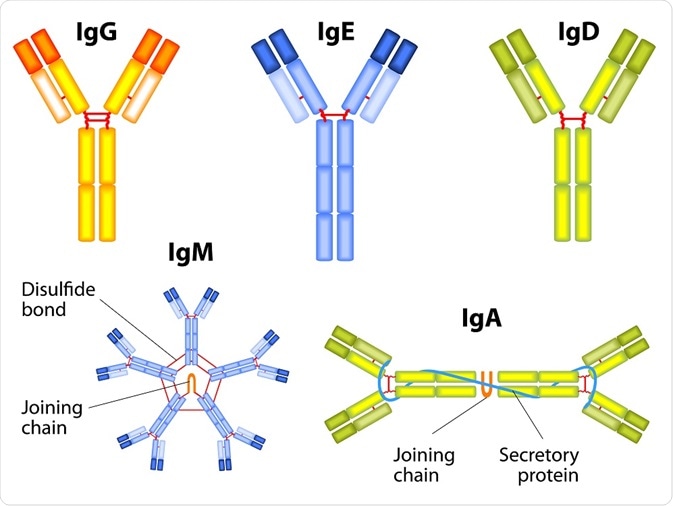
IgG
This isoform accounts for 70–75% of all human immunoglobulins found in the blood. Depending on the size of the hinge region, the position of disulfide bonds, and the molecular weight of the antibody, IgG can be further divided into 4 subclasses: IgG1, IgG2, IgG3, and IgG4.
In general, proteins are responsible for triggering IgG1 and IgG3 production, whereas IgG2 and IgG4 typically respond to foreign polysaccharides. IgG is the main component of the humoral immune system (immune response initiated by macromolecules present in the extracellular fluid) because of its abundance.
Due to its small size (monomeric) and high diffusibility, IgG is the prevalent type in the extracellular fluid that binds Fc receptors on phagocytic or other lytic cells and initiates the antibody-dependent cell-mediated cytotoxicity (ADCC) response – a cell-mediated defense mechanism wherein effector cells (phagocytes) destroy the target cell.
In addition, IgG triggers phagocytosis to initiate opsonization reaction – a process used to destroy foreign particles (e.g. bacteria) through phagocytosis. Apart from these functions, IgG is the only antibody that can cross the placenta and provides passive immunity to the fetus and infants in the first few months of life.
IgM
IgM is the largest antibody and the first one to be synthesized in response to an antigen or microbe, accounting for 5% of all immunoglobulins present in the blood. IgM typically exists as polymers of identical subunits, with a pentameric form as the prevalent one.
In its pentameric form, five basic antibody units are attached by disulfide bonds. Other forms include secretory IgM, which is synthesized by glandular-associated B cells, and monomeric form, which is present in the B cell membrane and functions as a B cell antigen receptor.
Due to its large size, IgM is mostly intravascular and has a lower affinity for antigens. However, since pentameric IgM has 10 antigen binding sites, it has higher avidity (overall binding strength) for antigens than IgG and acts as an excellent activator of the complement system and agglutination.
IgA
It accounts for 10–15% of all immunoglobulins and is prevalent in serum, nasal mucus, saliva, breast milk, and intestinal fluid. It has two subtypes namely IgA1 and IgA2, which mainly differ in terms of their hinge region characteristics. At mucosal surfaces, IgA provides the primary defense against inhaled and ingested pathogens.
IgE
IgE is the least prevalent one, with a serum concentration 10,000 times lower than IgG. However, the concentration of IgE increases significantly in allergic conditions, such as bronchopulmonary aspergillosis, and parasitic diseases, such as schistosomiasis.
In response to pathogens, IgE binds to mast cells via specific receptors, followed by pathogen-mediated cross-linking of these receptors (degranulation). This causes recruitment of eosinophil at the site of infection and destruction of pathogens via ADCC-type mechanisms.
IgD
IgD functions as a B cell antigen receptor and may participate in B cell maturation, maintenance, activation, and silencing. Although the exact function is still unclear, IgD may be involved in humoral immune responses by regulating B cell selection and homeostasis.
Camelid antibodies
Camelid (single-domain) antibodies, commonly known as nanobodies, are composed of only heavy chains (no light chains). These antibodies were discovered in 1989 following the analysis of total and fractionated IgG molecules in the serum of a camel.
In Camelid antibodies, the antigen recognition site is composed of a single variable domain. These antibodies are potential candidates to be used in antibody-based therapies because of some special characteristics, such as smaller size, good solubility, high thermostability, high affinity and specificity, low immunogenicity, and higher tissue penetration rate.
Further Reading