Opsonization is the mechanism by which targeting of particles for destruction through phagocytosis becomes enhanced. Opsonins are molecules that mark foreign particles for phagocytosis. Phagocytosis is the cellular process for removes pathogens and dead or dying cells.
Opsonization is the second step of phagocytosis, with chemotaxis first causing the recruitment of the phagocyte towards the site of infection or cell death. Opsonization, or the attachment of opsonins, then makes the pathogen more visible to the phagocyte, and the opsonized pathogen is then ingested by the phagocyte before intracellular destruction through digestion.
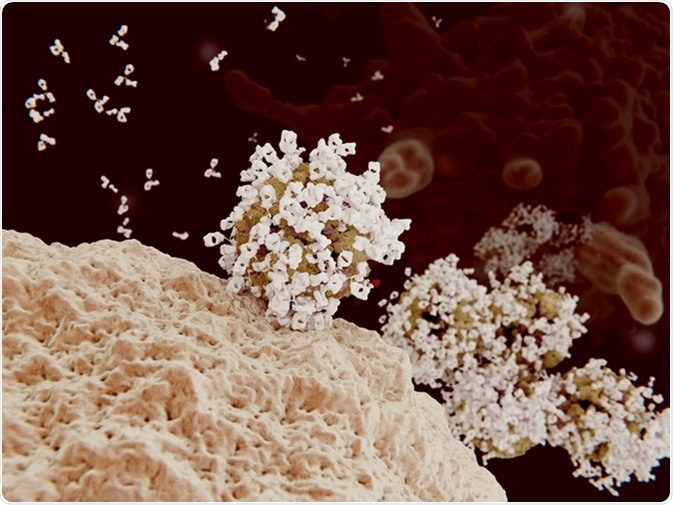
Antibody opsonization of influenza viruses. 3D rendering. Viruses coated with antibodies can't penetrate into their target cells and are engulfed and destroyed by a macrophage (background cell). Image Credit: Juan Gaertner / Shutterstock
Mechanism of Opsonization and Types of Opsonins
Opsonization occurs through the binding of an opsonin to an epitope of the pathogen or dead cells. Immune cells and pathogens all have negatively charged cell membranes. This causes the phagocyte and pathogen to be repelled away from each other. The opsonin molecule overcomes the repellant force of the negative charges through the interaction between the opsonin and the cell surface receptors on the immune cells. Specific antibodies can act as opsonins as well as compliment proteins of the innate immune response, and circulating proteins secreted from pattern recognition receptors can also be opsonins.
Antibodies
Antibodies are proteins made by immune cells which target specific molecules. These molecules can range from proteins present on the surface of bacteria, molecules secreted by bacteria or molecules present on host cells. These proteins are Y-shaped, and the “branches” are where the recognition occurs. Antibodies facilitate phagocytosis as the “stalk” of the antibody molecule is recognized by the phagocyte.
The Compliment System and Opsonization
The compliment system is a part of the innate immune response that bridges the innate (non-specific) and adaptive (specific) immune responses. The system is comprised of distinct plasma proteins that facilitate opsonization to reduce inflammation and remove pathogens. The compliment molecules C3b, C4b and C1q can all serve as opsonins.
Opsonization of Pathogens by Complement Proteins
The compliment system can lead to the removal of pathogens through three pathways:
- The classical pathway.
- The alternative pathway.
- The mannose-binding lectin pathway.
The classical pathway is activated by the binding of C1q to antigen-antibody complexes, thus forming a link between innate and adaptive immunity. C1q can also bind directly to the pathogen surface in the absence of an antibody. The C1q protein is a part of the C1 complex which acts on C4 and C3 molecules in a series of cleavage reactions to produce the classical C3 convertase, made up of components including C4b and C3b.
The alternative pathway involves the spontaneous activation of a compliment component binding to the surface of the pathogen. It requires factors such as B and D interacting with each other and C3b to form the alternative C3 convertase, C3bBb.
The mannose-binding lectin pathway begins with the binding of mannose-binding lectin (MBL) to mannose-containing carbohydrates on the pathogen. This leads to the production of MBL-associated serine proteases which in turn activate C4 and C3 to form the classical C3 convertase.
All three pathways converge at this point with C3 convertase cleaving C3 to produce C3b that binds to the pathogen cell membrane and opsonizes the pathogen.
Opsonization of Apoptotic Cells
The controlled death of cells through apoptosis and the clearance of these dead cells are important processes for preventing the accumulation of dead cells during inflammation. Poor removal of dead cells can lead to an active immune response that aggravates chronic inflammation. Opsonization removes apoptotic cells via soluble innate pattern-recognition proteins (sPRPs) that are able to recognize a diverse range of surface ligands on apoptotic cells. These circulating proteins include pentraxins, collectins and ficolins.
Phosphatidylcholine (PC) is a pentraxin molecule that is hydrolyzed in the later stages of apoptosis to form lysophosphatidylcholine (lysoPC), exposed on the surface of the dying, or apoptotic, cell. The soluble lysoPC acts as a chemo-attractant so the phagocyte can easily recognize the late apoptotic cell. Ligands on the dead cell act as a signal for the phagocyte to begin ingestion, with vital cells not presenting the same signals.
Sources
- Roos, A. et al. 2004. Mini‐review: A pivotal role for innate immunity in the clearance of apoptotic cells, European Journal of Immunology, 34, pp. 921-929. https://onlinelibrary.wiley.com/doi/abs/10.1002/eji.200424904
- Markiewski, M.M. & Lambris, J.D. 2007. The Role of Complement in Inflammatory Diseases From Behind the Scenes into the Spotlight, American Journal of Pathology, 171, pp. 715-727. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1959484/
- Janeway, C.A. Jr. et al. 2001. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The complement system and innate immunity. https://www.ncbi.nlm.nih.gov/books/NBK27100/
- Litvack, M.L. & Palaniyar, N. 2010. Soluble innate immune pattern-recognition proteins for clearing dying cells and cellular components: implications on exacerbating or resolving inflammation, Innate Immunity, 16, pp. 191-200. https://www.ncbi.nlm.nih.gov/pubmed/20529971
- Zhang, Y. et al. 2010. Coordination of Fc receptor signaling regulates cellular commitment to phagocytosis, PNAS, 107, pp. 19332-19337. http://www.pnas.org/content/107/45/19332
- British Society of Immunology: Compliment System. www.immunology.org/.../complement-system
Further Reading