Dr. Fay Saunders, UK Head of Upstream Mammalian Cell Culture, Process Development at FUJIFILM Diosynth Biotechnologies, shares her experience of using Cyto-Mine® in cell line development and recombinant protein expression.
FUJIFILM Diosynth Biotechnologies is an example of a contract development and manufacturing organization (CDMO). These organizations deliver essential support services for pharmaceutical and biopharmaceutical companies that work within an extremely competitive environment.
The expertise provided by FUJIFILM Diosynth Biotechnologies in cell line and process development enables more rapid progress of biotherapeutic molecules into clinical development to create stable mammalian cell lines with high-quality recombinant protein products at industry-leading concentrations.
FUJIFILM’s stable mammalian expression system, Apollo™ X, used together with the emerging technology of Sphere Fluidic’s Cyto-Mine® has transformed the organization’s approach to cell line development.
By substantially reducing timelines, FUJIFILM can quickly transition from the initial transfection phase to the delivery of highly productive research cell banks within approximately 10 weeks. The organization aims to deliver the most efficient and robust cell lines that display the best possible productivity.
This can be difficult as the industry is currently seeing a shift in focus in the biological medicine field, away from standard therapeutic monoclonal antibodies toward antibody fragments and bispecific antibodies that are more challenging to express in cellular systems.
This is a commercially appealing and fast-paced part of the drug development industry. Timelines for cell line provision are experiencing growing scrutiny as companies are in competition for their share of the marketplace.
To complicate matters further, evidence of monoclonality must be provided for all cell lines producing biotherapeutic molecules if they are to obtain approval from industry regulators such as the Food and Drug Administration (FDA).
Companies must provide evidence that the cell line producing the therapeutic molecule originates from a single parent cell.
Companies must be able to prove that the therapeutic molecule’s cell line is derived from a single parent cell. Time-consuming and low-throughput conventional screening techniques – such as colony picking and limiting dilution cloning - do not allow individual cells to be visualized, and monoclonality can be challenging to demonstrate using these methods.
Higher throughput screening methods, including fluorescence-activated cell sorting (FACS), deliver higher efficiency, but they can be difficult to utilize. These methods can only detect membrane-bound antibodies and may compromise fragile cell lines as suspensions are transported through the system under pressure.
Time-consuming and low-throughput conventional screening techniques (e.g. colony picking and limiting dilution cloning) do not allow individual cells to be visualized and monoclonality can be difficult to demonstrate using these methods.
Dr. Fay Saunders, FUJIFILM Diosynth Biotechnologies

Image Credit: Sphere Fluidics
Enhancing quality and efficiency with single-cell technology
Integrating Cyto-Mine® within cell line development processes at FUJIFILM Diosynth Biotechnologies has eliminated bottlenecks and considerably shortened timelines, which are subsequently only constrained by cell doubling times.
Employing this technology has enabled the implementation of a novel single-step cloning technique, replacing the organization’s two-step process.
Pools of transfected cells are encapsulated as single cells within individual picolitre-sized aqueous droplets in a biocompatible carrier oil (picodroplets). Each picodroplet delivers a defined and supportive environment that preserves the viability of cells, as shown in Figure 1.
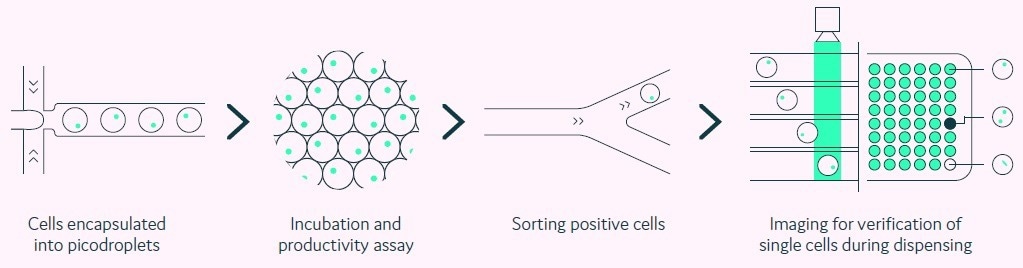
Figure 1. Integration of productivity assay screening, sorting, isolation and verification using a fully automated picodroplet microfluidic process.Image Credit: Sphere Fluidics
A fully automated process is utilized to quickly screen and characterize single cells and their secreted proteins. Formerly, screening demanded lengthy manual procedures and analysis methods.
However, due to the miniaturization of its assays within an integrated, multiple-step platform, Cyto-Mine® enables analysis to be conducted within a single day rather than weeks.
Moreover, screening of approximately 200,000 cells is now possible in a matter of hours, compared to approximately 10,000 cells using multistep manual techniques.
Utilizing this streamlined approach has enabled the identification of highly productive recombinant cell lines within 10 weeks, as displayed in Figure 2. Importantly, the Cyto-Mine® system can be utilized to visualize single cells, which provides evidence of monoclonality to meet the regulatory requirements.

Figure 2. Example timeline highlighting the streamlining of workflows using mammalian cell line engineering technologies in combination with single-cell picodroplet innovations. Image Credit: Sphere Fluidics
FUJIFILM’s workflows are significantly more efficient as cellular screening is performed early in the process, enabling the selection of the highest-performing cells (expressing the protein of interest) to progress for development.
This brings advantages regarding the use of resources (e.g., reagents) and lab space, which is optimized because of the ability to have multiple screening, sorting, and validation steps in a single machine. Wastage will also decrease over time.
This new approach allows scientists in the lab to be freed from laborious manual procedures, enabling them to apply their skills and expertise to other valuable aspects of their work. The automated platform is user-friendly, with minimal training required ahead of use.
Applications within antibody discovery may enable the rapid screening of large molecular libraries to identify high-specificity antibodies that recognize novel cellular targets. This would offer an exceptional starting point for later cell line development.
There are considerable possibilities for further innovation with this technology, and FUJIFILM Diosynth Biotechnologies looks forward to exploring these opportunities provided by Cyto-Mine®.
The advantages of picodroplet technology include:
- Miniaturization and integration of multiple processes/pathways
- Streamlined and simplified processes
- Assured monoclonality
- Accurate screening of large cellular populations (200,000 individual cells)
- Reduced timelines/workflows
- Increased efficiency
- Automated processing and greater freedom from manual tasks

Image Credit: Sphere Fluidics

 Download the full interview
Download the full interview
Acknowledgments
Produced from materials originally authored by Sphere Fluidics and Dr. Fay Saunders, UK Head of Upstream Mammalian Cell Culture, Process Development at FUJIFILM Diosynth Biotechnologies.
About Sphere Fluidics
Our Vision
Our philosophy is simple. We combine our knowledge and resources to help you find rare and valuable biological variants, while helping you to save time, reduce costs and stay a step ahead of the competition.
Our novel single cell analysis systems offer the rapid screening and characterization of single cells. These systems are underpinned by our patented picodroplet technology, specifically designed to increase your chances of finding that rare ‘one-in-a-billion’ molecule or cell that could be an industry blockbuster.
We understand that time is of the essence. That’s why our technologies boost throughput and assay sensitivity across a range of applications. Most importantly, our flexible systems evolve alongside your changing research needs, providing an adaptable platform that helps you to meet your goals.
Our History
Founded in 2010, Sphere Fluidics is an established Life Sciences company, originally spun out from the University of Cambridge. We initially developed 25 patented products – biochips and specialist chemicals – which currently assist hundreds of customers globally with their research.
We initially focused on producing novel biochip systems and providing R&D services. We have since extended our expertise and are developing a technology platform that enables discovery in a range of growing markets through single cell analysis. Our systems make the development of new biopharmaceuticals faster and more cost-effective, improve monoclonal antibody screening, cell line development, and overall research efficiency in a number of other applications including synthetic biology, single cell diagnostics, prognostics and single cell genome editing.
The Cyto-Mine® Single Cell Analysis System is our flagship product – the first integrated, benchtop system to automatically analyse, sort and dispense millions of individual cells in just a single day.
Our Partnerships
We value and are always open to discussing new collaborative, successful and innovative academic and industry partnerships to further develop and improve our single cell technologies.
Our Technology Access Programmes and Collaborative Services exist to enable academic researchers and companies alike to tap into our application-specific expertise through direct partnerships.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.